Types of semiconductors: N type Semiconductor and P Type Semiconductor
Table of Contents
Semiconductors, Introduction:
Types of Semiconductors- In this article, we will study in detail about the types of Semiconductors. What are N type and P type semiconductors? What is Valence Band and Conduction Band? How the Intrinsic Semiconductor and Extrinsic Semiconductor are made? You will get answers to all of these questions.
Semiconductors are divided into two types.
- Intrinsic semiconductor or pure form
- Extrinsic semiconductor impure form
- Intrinsic semi conductor or pure form:
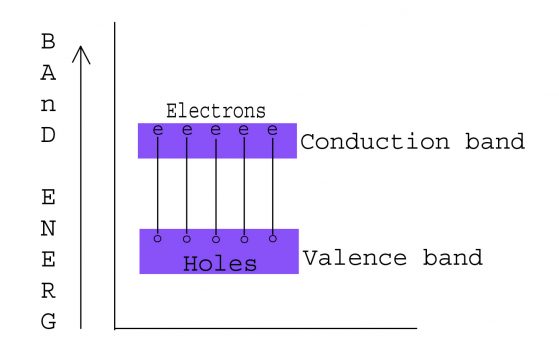
A semiconductor in its extremely pure form is called intrinsic or pure semiconductor. A semiconductor is not truly intrinsic unless its impurity content is less than 1 part impurity in 100 million parts of semiconductor. As at Ok temperature no conduction is there but when temperature is raised some electrons near the top of valence band are thermally ionized in the level of conduction band. The conduction band now contains a few electrons and the valence band will have few missing electrons and that are called holes. So thermal ionization will take place and it will create holes electrons pair as shown.
This type of generation is called thermal generation.(Thus it is concluded that semiconductor conducts current for an intrinsic semiconductor consist of movement of electrons and holes ( in pair ) in opposite direction in the conduction and valence band respectively). For an intrinsic semiconductor the number of conduction electrons is equal to the number of holes. The energy band diagram of an intrinsic semi conductor is given as.
Here FERMI level lies in the middle of the forbidden energy gap because the number of conduction electrons and holes are equal. FERM level is defined as the energy which correspond to the center of gravity of conduction electrons and holes weighted according to their energy.
Extrinsic semiconductor:
Those intrinsic semiconductors to which some suitable impurity (doping agent) has been added in extremely small amount, that is about 1 part in 108 are called extrinsic or impure semiconductor.
OR
(The dope semiconductors are called extrinsic semiconductor). The dope agents are pentavalent or Trivalent atoms (B, Sb, Ar, P). The pentavalent atom have five valence electrons. These types of doping agents are called as pentavalent impurity.
Out of five electrons of pentavalent, four are combined with four electrons of Ge or silicon to complete its octed. The 5th electron remain free; by doing the process again and again we can get many and many free electrons. The pentavalent atom donate one electron or it contribute one electron, that is why pentavalent atom is known as DONAR atom.
The trivalent atoms ( Gallium Ga, indium In , Aluminum Al, boran B)( Have three valence electrons). These type of doping agents are called as trivalent impurity. Four electrons of germanium or silicon will make three covalent bonds with three electrons of trivalent. To complete its octed, there is deficiency of one electron or there is vacancy for one electron. As deficiency of electron is denoted by hole or +Ve, doing the same process again and again there will be abundance of hole or many holes will be there. As these type of atoms accepts one electron from nearby atom. Hence the trivalent doping atoms are known as acceptor atom. Depending on the type of doping material used, extrinsic semiconductors are sub divided in two types.
- N type semiconductor
- P type semiconductor
N type semiconductor:
This type of Semiconductor is obtained when pentavalent impurity is added to pure Ge crystal. Each pentavalent atom forms four covalent bonds with the four electrons of Si or Ge. (A bond formed by mutual sharing of electrons). Out of 5-electrons of pentavalent atom four are combined with the four electrons of Ge or Silicon, the fifth electron is super-fluous(extra), and is loosely bound with the nucleus of pentavalent atom. So this electron is also called as free electron. It can be easily excited from the valence band to the conduction band. By the application of external energy (that may be electrical energy or thermal energy). The antimony (Sb) atom is called Donor impurity because it donates one electron to the nearby atom as shown below.
So the addition of Sb greatly increases the number of conduction electrons. Hence concentration of the electrons increases and exceeds the concentration of holes in the valence band. Thus the Fermi level shifts upward towards the bottom of the conduction band as shown below.
The 5th electron (free electron) has an energy level which is called Donor Level. This energy level is 0.01 e.v for Ge and it is .054 ev for Si.
It is concluded that in N type semiconductor electrons are the majority carriers, while holes are the minority carriers.
Note that in DoNor the letter “N” is for N type extrinsic semiconductor and “N” is for Negative charge carriers.
P type of semiconductor:
This type of semiconductor is obtained when trivalent impurity (GA, Indium, Aluminum, B) are added to a pure Ge crystal. The three valence electrons of “B” form three covalent bonds with four surrounding Ge, but the fourth bond remains incomplete which will rise a hole and that will be the vacancy for an electron as shown in figure given below.
In the fig it is shown that there is vacancy for one electron, so it will accept an electron from the nearby atom. “B” is called acceptor impurity, repeating the process again and again there will be many and many holes. Note that in acceptor letter “P” is for P-type extrinsic semiconductor and also “P” for +Ve charge carrier. In this type semiconductor the conduction is by means of holes. Note that hole current flow more slowly than electron current. Here holes for the majority carrier where electrons constitute minority carriers. Concentration of holes in the valence band is more than the concentration of electrons in the conduction band. Hence FERMI level shift near to the valence band and the acceptor level lies just above the Fermi level. As shown in the figure given below.
Conduction taken place due to the movement of conduction holes at the top of valence band and the acceptor level immediately accept electron from the valence band.